electron configuration al|full electron configuration al : Cebu Click on above elements (in Periodic table) to see their information or Visit . HOW TO WIN AT SLOTS. Slot machines are games with odds based in math, just like all other casino games. But few players understand just how those odds work, and whether they can do anything to improve their odds. The objective of this chapter is to introduce you to how slot machine odds work and what that means to your chance to win at slots.
PH0 · which element has this electron configuration
PH1 · full electron configuration of aluminum
PH2 · full electron configuration al
PH3 · electronic configuration of 1 to 20 elements
PH4 · electron configuration periodic table
PH5 · electron configuration for al+3
PH6 · electron configuration calculator
PH7 · electron config chart
PH8 · Iba pa
Estás a punto de entrar en un sitio web que contiene material explícito (pornografía). Sólo debes acceder a este sitio web si tienes al menos 18 años o la edad legal para ver dicho material en tu jurisdicción local, lo que sea mayor.
electron configuration al*******March 23, 2023. Electron configuration chart of all Elements is mentioned in the table below. The Shorthand electron configuration (or Noble gas configuration) as well as Full electron configuration is also mentioned in the table. Atomic no.Click on above elements (in Periodic table) to see their information or Visit .Therefore the Aluminium electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 1. Video: Aluminium Electron Configuration Notation. The configuration notation provides an .
Electron Configuration of Aluminum. To find the electron configuration of an atom, you first need to know the number of electrons that it has. Since aluminum's . Aluminum Electron Configuration - YouTube. Wayne Breslyn. 746K subscribers. Subscribed. 657. 108K views 10 years ago. A step-by-step description of how to write the electron configuration.The Electron configuration of aluminum is 1s22s22p63s23p1. Aluminum is one of the elements that make up the periodic table, which is distinguished by its symbol Al, and its atomic number 13. This element, which has 13 .What is the electron configuration and orbital diagram of: Na + P 3– Al 2+ Fe 2+ Sm 3+ Solution. First, write out the electron configuration for each parent atom. We have .What is the electron configuration of: (a) Na + (b) P 3– (c) Al 2+ (d) Fe 2+ (e) Sm 3+ Solution First, write out the electron configuration for each parent atom. We have chosen to show .
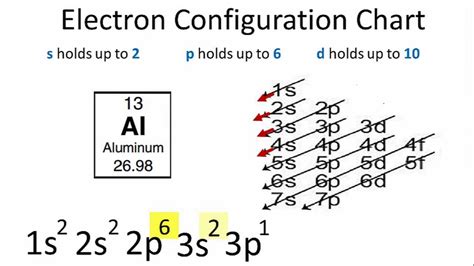
Updated on February 01, 2021. The electron configuration of an atom of any element is the of electrons per sublevel of the energy levels of an atom in its ground state . This handy chart compiles the electron .full electron configuration al Updated on February 01, 2021. The electron configuration of an atom of any element is the of electrons per sublevel of the energy levels of an atom in its ground state . This handy chart compiles the electron .electron configuration al This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. Typically, you need at .
The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon . What is the electron configuration of: (a) Na + (b) P 3– (c) Al 2+ (d) Fe 2+ (e) Sm 3+ Solution First, write out the electron configuration for each parent atom. We have chosen to show the full . A step-by-step description of how to write the electron configuration for Aluminum (Al). In order to write the Al electron configuration we first need to kn. The shorthand electron configuration for Aluminum is [Ne] 3s 2 3p 1. The electron configuration for the Aluminum ion (Al 3+ ) is 1s 2 2s 2 2p 6. The number of valence electrons available for the Aluminum atom is 3. Aluminum is situated in Group 13th or 3A and has an atomic number of 13. The third major category of elements arises when the distinguishing electron occupies an f subshell. The first example occurs in the case of the lanthanoids (elements having atomic numbers between 57 and 71).The lanthanoids have the general electron configuration [Kr]4d 10 4f i 5s 2 5p 6 5d 0 or 1 6s 2. where i is a number between 0 and .
Assigning Electron Configuration . We write electronic configurations by following the aufbau principle (from German, meaning “building up”). First we determine the number of electrons in the atom; then we add electrons one at a time to the lowest-energy orbital available without violating the Pauli Exclusion Principle .That is, recognizing that each .The easiest way to create electron configurations is using an electron configuration table, which is a way of writing down the various orbitals available to electrons. This table is easy to remember, and it makes it possible to generate the electron configuration table for any given element. It looks something like this.
1 Answer. Aluminium has atomic number 13 so the full electron configuration will be: 13Al 1s22s22p63s23p1. A shorthand way of writing this is to use the preceding noble gas configuration by putting its symbol in square brackets in front of the valence electrons. In this case that is neon which is: Introduction. The electron configuration is the standard notation used to describe the electronic structure of an atom. Under the orbital approximation, we let each electron occupy an orbital, which can be solved by a single wavefunction. In doing so, we obtain three quantum numbers (n,l,m l), which are the same as the ones obtained from . An atom's electron configuration describes the way its electrons fill sublevels when the atom is in its ground state. Atoms seek the most stable electron configuration, so sublevels are half-filled or fully-filled .
The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = +1 2 m s = + 1 2 ).Introduction to electron configurations. Google Classroom. About. Transcript. Electron configurations describe where electrons are located around the nucleus of an atom. For example, the electron configuration of lithium, 1s²2s¹, tells us that lithium has two electrons in the 1s subshell and one electron in the 2s subshell. Created by Sal Khan.
The electron configuration states where electrons are likely to be in an atom. If you don’t have a chart, you can still find the electron configuration. Use the element blocks of the periodic table to .

Electron Configurations. The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. Commonly, the electron configuration is used to .
Al (Aluminum) is an element with position number 13 in the periodic table. Located in the III period. Melting point: 660.5 ℃. Density: 2.7 g/cm 3 . Electronic configuration of the Aluminum atom: 1s 2 2s 2 2p 6 3s 2 3p 1Reduced electronic configuration Al: [Ne] 3s 2 3p 1. Below is the electronic diagram of the Aluminum atom Distribution of .
In the periodic table, the elements are listed in order of increasing atomic number Z. Electron configuration of Aluminium is [Ne] 3s2 3p1. Possible oxidation states are -2; -1; +1; +2; +3. An aluminium atom has 13 electrons, arranged in an electron configuration of [Ne] 3s2 3p1, with three electrons beyond a stable noble gas configuration.
Add an electron to the anion electron configuration. For example, the ground state electronic configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. For Cl −, it will be 1s²2s²2p⁶3s²3p⁶. Remove the outermost electrons in the cation, e.g. electron configuration for Mg 2+ will be 1s²2s²2p⁶.electron configuration al full electron configuration alElectron Configuration -The Electron Configuration of an Element Describes how Electrons are Distributed in their Atomic Orbitals. In Electronic Configuration electrons are arranged in various shells, Subshell and Orbital by following certain rules. To Learn how to Write Electronic Configurations, Detailed Explanation, Filling of orbital with FAQs, .The electron configuration of the aluminum shows that it has three unpaired electrons in the last shell(3s 1 3p x 1 3p y 1). Therefore, the valency of aluminum is 3. How many valence electrons does aluminum ion(Al 3+) have? After the electron configuration, the last shell of the aluminum atom has three electrons. In this case, the valency of .
Get Arranged Lyrics & Video for "ECHO In JESUS Name Lyrics by Charity Gayle" From ENDLESS PRAISE Album. Released September 10th 2021.
electron configuration al|full electron configuration al